Pre Award
We encourage all faculty to apply for funding opportunities available from external sponsors, internal programs, and philanthropy, as well as through collaborations with other investigators. Below you will find resources available to faculty researchers in the Department of Surgery (DOS). As a reminder, all applications must be reviewed and approved by the DOS Grants Administration Office and Partners Research Management with the exception of internal funding opportunities (e.g., Brigham Research Institute, Osteen, and Beal Fellowships).
Brigham Research Institute
The BRI offers a wide variety of internal funding opportunities. Since these are internal, they do not need to be reviewed by the DOS Grants Administration Office or Partners Research Management.
BWH Development/Philanthropy
There are several ways to receive gift support. Please contact the BWH Development office directly if you are interested in learning more about general gift planning, how to participate in an event, and how to start a fundraiser.
Department of Defense Congressionally Directed Medical Research Programs (CDMRP)
The CDMRP fills research gaps by funding high impact, high risk, and high gain projects that other agencies may not venture to fund. Note: All pre-applications must be submitted electronically to the Electronic Biomedical Research Application Portal (eBRAP) https://ebrap.org. Full applications must be submitted electronically to the Grants.gov website https://grants.gov. If you do submit a pre-application, we recommend that you start on the full application submission process even before receiving the formal invitation.
Foundation Grant Funding
Private foundations are established to support charitable endeavors; they award grants to institutions that align with their own mission. Below is a select list of foundations that have funded past investigators in the department. Please visit each foundation’s/society’s direct website for more information on available funding opportunities.
- American Cancer Society
Institutional Research Grants
Index of Grants - American College of Surgeons
American College of Surgeons Research Scholarships, Fellowships, and Awards
Resident Research Scholarship
Wyeth-Ayerst Resident Research Scholarship
Faculty Research Fellowship
George H.A. Clowes Jr., MD, FACS, Memorial Research Career Development Award - American Surgical Association
ASAF Fellowship Research Award - Association for Academic Surgery
Roslyn Faculty Research Award - Association for Surgical Education Foundation
Center for Excellence in Surgical Education, Research and Training Grant - Society of Surgical Oncology
Clinical Investigator Awards - Society of University Surgeons
Junior Faculty Award
National Institutes of Health (NIH) Federal Funding
NIH-funded research has led to breakthroughs and new treatments, helping people live longer, healthier lives, and building the research foundation that drives discovery. Parent announcements are broad funding opportunity announcements allowing applicants to submit investigator-initiated applications for specific activity codes. They are open for up to 3 years and use standard due dates.
Not all NIH Institutes and Centers participate in all parent announcements. Before submitting your application, make sure the NIH Institute or Center that might be interested in your research is listed as a participating organization in the announcement.
Harvard Catalyst
Harvard Catalyst is a pan-University collaborative effort committed to harnessing the human, technological, and fiscal resources of Harvard and its academic healthcare centers to reduce the burden of human illness. The Harvard Catalyst offers seed funds in the form of Pilot Grants and Prizes to foster cross-institutional and cross-disciplinary collaboration. Sign up for the Harvard Catalyst newsletter to be alerted of upcoming funding opportunities only available to Harvard-affiliated hospitals.
PIVOT
PIVOT is a searchable database of funding opportunities. You will need to sign up (use your Partners or Harvard email address and select Harvard University as your institution when prompted) to be able to search for funding applications using a variety of parameters as well as create alerts. Please use the Pivot User Guide for more information.
Proposal Initiation
- Verify eligibility.
- Notify Grants Administrator (GA) of intent to submit proposal ASAP, and no later than 15 business days before the agency deadline.
- Complete DOS proposal intake form via Redcap <link>
- Provide GA with link to and/or PDF of funding opportunity and guidelines.
- Review the guidelines specified in the funding opportunity announcement (FOA).
Proposals with Subcontract(s)
- Notify GA of institution(s) involved and the administrative contact for each institution, and copy your DOS GA, if applicable.
- Provide GA with details for each subcontract’s allocation (personnel effort, non-personnel expenses).
- Assist with following up with subcontract PI(s) when needed to ensure documents arrive in a timely manner.
Proposal Budget and Budget Justification
- Provide reasonable effort levels for personnel and details of non-personnel expenses.
- Provide language for the budget justification detailing the work performed by all personnel. relative to the effort requested.
- Provide justification for all non-personnel expenses.
- Provide sundry fund for proposals that are not listed on the Partners indirect costs (IDC) exemption list and:
- Do not allow IDC;
- Do not allow use of negotiated rates;
- Do not meet the IDC floor.
- Provide sundry fund for proposals that include voluntary cost-share, such as:
- Use of the NIH salary cap on Department of Defense proposals;
- Not charging PI salary to a proposal because they have protected research time.
Proposal Components
- Prepare all required components and send to GA in the correct format by the internal deadline.
- Provide GA with letters of support from collaborators/consultants.
- Provide GA with letters of commitment from the department chair/division chair and institution when required.
Proposal Approval and Submission
- Complete conflicts of interest in Insight as soon as request is received.
- Approve proposal in Insight as soon as request is received (the proposal will not move to the next level until after the PI has approved).
- Review final version of proposal for completeness and accuracy before submission.
- For NIH proposals, review assembled proposal in Grants.gov for completeness and accuracy.
Proposal Initiation
- Create agreement in Insight.
- Review proposal intake form and notify PI of any additional information needed for the proposal.
- Initiate electronic conflicts of interest process in Insight and ensure that this is completed by all key personnel before submission.
Proposals with Subcontract(s)
- Send budgetary details and list of required documents to subcontract institutions. Ensure the deadline provided to subcontract institutions allows for enough time for us to meet internal and Partners deadlines.
- Follow up with contacts at subcontract institutions to ensure that documents are received in a timely manner.
- Review documents received from subaward institutions for completeness and accuracy.
Proposal Budget and Budget Justification
- Conduct preliminary review prior to sending to proposal budget and justification to RM.
- Ensure that effort levels are reasonable based on proposed work.
Proposal Components
- Ensure that all required components of the proposal have been submitted to RM for review.
- Ensure that Sponsor Guidelines regarding formatting of the proposal have been adhered to.
Proposal Approval and Submission
- Once RM has approved the proposal and signed off, obtain final approval from the PI to proceed with submission.
Proposal Initiation
- Assist in requesting eRA Commons and ORCiD accounts.
Proposals with Subcontract(s)
- Review subaward budgets and documents for accuracy and compliance.
Proposal Budget and Budget Justification
- Conduct initial review of budget and budget justification.
- Ensure that budget and budget justification are in compliance with agency guidelines.
- Provide feedback to GA.
- Ensure that effort levels are reasonable given the PI’s already committed and proposed effort.
- Review any changes and ensure that all questions/feedback are addressed.
- Provide final sign-off on budget and budget justification.
Proposal Components
- Conduct initial review of all proposal components (scientific, budgetary, administrative).
- Notify GA of all necessary changes to ensure that components are in compliance.
- Review updated components/documents.
Proposal Approval and Submission
- Conduct final review of the proposal.
- Sign as the Authorized Business Official.
- Submit proposal in eRA Commons (for NIH proposals) or other agency mechanism/website.
- Forward submission confirmation emails from the sponsor to the PI and GA.
For faculty who are new to writing proposal content for grant applications, we encourage you to explore the following guides and resources. Your research mentor(s) should also be able to provide expert guidance on writing a strong grant proposal. Please reach out to Jamie Fu if you are interested in seeing sample NIH-funded applications of previous DOS submissions.
DOS Research Development Team[TBA]
The DOS Research Development Team is a new team available to the department’s faculty researchers. The team consists of two PhD scientists, one biostatistician, and one medical editor. The goal is to coordinate and manage complex investigator, program project, and training grant proposals (e.g., K, R, T32, or STARR grant proposals).
Brigham Research Education (BRE) Program
The Research Education Program at BWH provides a centralized resource to promote and facilitate research education and works closely with groups across the research community. Grant writing resources include the Grant Review and Support Program (GRASP), Elements of Grant Writing, and Tips for Grant Writing.
BWH Center for Clinical Investigation (CCI)
CCI offers research space and services to support BWH investigators with an IRB-approved protocol.
BWH Office for Research Careers (ORC)
Brenda Birmann, part-time grant editor with the ORC, provides editing services for grant proposals. Brenda assists with English language, grammar, and writing style, and ensures formatting and content is in line with NIH guidelines. You may sign up for either a ‘Specific Aims only’ or ‘Full Proposal’ slot in the months preceding your submission deadline.
National Institutes of Health (NIH) Center for Scientific Review
The NIH Center for Scientific Review provides resources to assist in developing a strong application that allows reviewers to better evaluate the scientific merit of your proposal.
Requesting a new* eRA Commons account/username:
*If you have an existing eRA Commons account/username that you need to transfer, or multiple accounts that need to be merged, please refer to the “Transferring or Merging eRA Commons Account(s)” link.
An eRA Commons account/username is required for:
Proposal Submission:
- Principal investigator/Project Director (PI)/PD, and any multiple PI/PDs (including fellowships);
- Sponsor on a fellowship application;
- Component leads on a multi-project application;
- Candidates for diversity supplements;
- Primary mentor on an individual mentored career development proposal;
- Anyone who will be working in ASSIST.
Progress Report:**
- PD/PI and any multiple PD/PIs;
- Undergraduate;
- Graduate student;
- Postdoctoral associates.
**Required for all personnel who participate in an NIH-funded project for at least one (1) person month, although it is recommended for all other project personnel.
PROCESS
PI/Research Personnel:
- Notify your grants administrator (GA) that you need an eRA Commons account set up.
- Provide requested information and/or respond to confirmation requests.
- If you already have an existing account (e.g., at your previous institution), please let your GA know so your institutional affiliation can be changed. A new account does not need to be created.
- If you have an existing account or multiple accounts (e.g., you created a new account when you moved to a different institution instead of transferring your existing account), you will need to submit a request for your account(s) to be transferred and/or merged. The process for transferring and merging accounts is detailed in a separate link.
- Once account is created, log in and complete your profile as soon as possible; incomplete profiles will delay submission of the proposal or progress report.
Grant Administrator (GA):
- Ensure that all required personnel have an eRA Commons user account/username as soon as you are notified of a proposal submission.
- Confirm with the PI/research personnel that they do not have an existing account. If they have an existing account, follow the procedure for transferring/merging eRA Commons accounts. This information is saved under a separate link in this section.
- Email the MGB pre-award administrator assigned to the PI/research personnel’s chief code requesting that an account be set up (make sure you copy the PI/research personnel on the email).
- Follow up with the MGB pre-award administrator if needed.
- Once you are notified that an eRA Commons username/account has been created for a PI or for research personnel, ask that they update their profile and make sure that they are affiliated with BWH. An incomplete or incorrect profile will delay submission of a proposal or progress report.
*This is for anyone who has an existing National Institutes of Health (NIH) Electronic Research Administration Commons (eRA Commons) account/username. If you are a new user, please see the link on requesting a new eRA Commons account under this section.
TRANSFERRING/MERGING eRA COMMONS ACCOUNTS
Principle Investigators (PIs)/Research Personnel
Transferring an account
- Email your grants administrator with your existing eRA Commons username and the institution with which it is affiliated.
Merging multiple accounts
- If you have multiple accounts (e.g., you created a new account when you moved to a different institution instead of transferring your existing account), you will need to submit a request for your accounts to be merged in eRA Commons. Instructions and screenshots can be found here.
Once you receive notification that your account has been transferred or that your accounts have been merged, please log in to verify and complete your profile as soon as possible. An incomplete profile will delay the submission of your proposal or progress report.
Grant Administrators
- For PIs/research personnel who need to transfer an account/username, email your Research Management Pre-Award Administrator and ask that the account/username be affiliated with BWH.
- If BWH is still not listed as the affiliated institution when you list the PI/research personnel on a proposal or progress report, email them and ask them to make sure that their profile has been updated to reflect BWH as their affiliated institution. They will need to do this by logging on and updating their eRA Commons profile.
An eRA Commons account/username is required for all personnel who participate in an NIH-funded project for at least one-person month, although it is recommended for all other project personnel.
As a reminder, all applications must be reviewed and approved by the DOS Grants Administration (GA) office and Partners Research Management (RM), with the exception of internal funding opportunities (e.g. Brigham Research Institute, Osteen, and Beal Fellowships). When your grant application is in its final form and ready for internal review, please follow the workflow outlined below.
- PI e-mails FINAL attachments to GA for 1st round review
- GA uploads to Insight and/or Sponsor website
- GA routes Insight record to Partners RM for internal review
- PI receives notification to e-approve in Insight
- Partners RM review complete
- GA and PI address comments
- PI reviews/ approves FINAL version via e-mail
- Partners RM submits federal applications on behalf of PI
- GA submits non-federal applications on behalf of PI
NATIONAL INSTITUTES OF HEALTH (NIH) NEW AWARD DEADLINES
If deadline falls on a weekend or holiday, it will move to the following business day
INTERNAL DEADLINE – 21 DAYS PRIOR TO SPONSOR DEADLINE
SUBCONTRACTED INSTITUTIONS – NOTIFIED 50 DAYS PRIOR TO SPONSOR DEADLINE
CYCLE I
CYCLE II
CYCLE III
Peri Award
Background
A Just-In-Time (JIT) is a request for additional documents from the National Institutes of Health (NIH) if a notice of award is likely. JIT requests include up-to-date Other Support pages, approval notices for institutional animal care and use committee (IACUC) and institutional review board (IRB) protocols pertinent to the proposal, and Human Subjects Education documents (i.e. CITI Training Documents). It should be noted that when “JIT” is listed in the “Action” column of your NIH eRA Commons account next to the award submission, this only indicates that your award has completed the peer review process and has received a score that may indicate possible funding. It is not until a formal request, via email, that the NIH will request JIT documents.
For additional information, proceed to the NIH JIT informational PDF at http://grants2.nih.gov/grants/peer/jit.pdf
Receiving a JIT Request
As mentioned above, a JIT request will be sent via email to the Principal Investigator (PI) and Mass General Brigham Research Management (RM). It is important that the PI forwards the JIT request to their Research and Grant Administrator within the Department of Surgery as soon as possible. JIT requests typically include a specific due date and often have very short turnaround time requests (often within 5 business days).
Standard JIT Requirements
- Other Support Pages
Other Support pages include all financial resources, whether Federal, non-Federal, commercial or institutional, available in direct support of an investigator’s research activities including but not limited to cooperative agreements, contracts, and/or institutional awards. Other Support also includes resources from all foreign and domestic entities such as financial support, laboratory space, or provision of high-value materials that are not freely available (e.g., biologics, chemical, model systems, technology, etc.).Training awards, prizes and gifts do not need to be included. Other Support pages are required for all Key Personnel in JIT submissions. It is not requested for individuals who were identified as Other Significant Contributors.Other Support includes active and pending support in calendar months. Your Grant Administrator and Mass General Brigham RM will review this information to confirm there is no scientific, budgetary or commitment overlap.Please note: You cannot have “zero/none” or “as needed” commitment on any active/pending support listed.Should your total committed effort exceed 12 calendar months, a justification/explanation on how effort will be adjusted should be included.
- Certification of IRB approval
This is required if the proposed project includes human subjects research. It should be noted that pending or expired approvals are not accepted. The NIH requires that all non-exempt human subjects research be reviewed and approved by an IRB. The IRB approval notice should indicate that the pending award is an approved source of funding for the IRB protocol.
- Certification IACUC approval
This is required if the proposed project includes research with live vertebrate animals. It should be noted that pending or expired approvals are not accepted. The NIH requires that all non-exempt human subjects research be reviewed and approved by an IACUC. The IACUC approval notice should indicate that the pending award is an approved source of funding for the IACUC protocol.
Other Information Requested by the Awarding NIH Institute/Center
The NIH may also request additional information on a case-by-case basis. These additional items will be included in the initial request email. Items that may be requested include a revised budget changes to the Human Subjects or Vertebrate Animals section of the application.
Review and Submission of JIT
The JIT package should be generated by the PI and the Research Administrator. The PI is responsible for uploading the JIT documents to the eRA Commons record within eRA ASSIST. Communication and review of the JIT by Mass General Brigham Research Management should be conducted by email. The PI and Research Administrator are responsible for coordinating the review and final submission with their Pre-Award Grants Manager at the Mass General Brigham RM level. Mass General Brigham RM has the responsibility of the final submission of the materials.
Overview
All Partners Investigators and Study Staff must complete a CITI Human Subjects Protection training course every three years. To begin you must complete one of the two CITI BASIC courses offered, preferably the Biomedical BASIC course. Once completed, your education requirement will be fulfilled for three years. After three years, you must take a refresher/continuing education course.
Account Registration
If you do not have a CITI account, please follow the instructions below to register and create a new account.
- Go to https://www.citiprogram.org and click the “Register” button.
- Select an institution (Massachusetts General Hospital or Brigham and Women’s Hospital), affirm that you agree to the terms and conditions and click “Continue.”
Note: Do not select the “Independent Learner” registration. - Enter in your personal information and click “Continue.”
- For your username, please use your PHS username and create a password. Note: If your PHS username is fewer than 4 characters, please put a “P” after the 3 characters of your username. You will also need to answer the security question.
- Continue to complete the rest of the registration screens. A few important notes are listed below:
- All fields marked with an asterisk must be completed.
- In Step 5, CEU credits are not given for CITI exams. Please check “no” on this screen when asked.
- In Step 7:
- click the box next to “Will you be working with humans (IRB courses)?”
- select “Biomedical Research” when asked to select a course most appropriate to your research activities.
- If you are requested to take the Good Clinical Practice Course, please click this option. This course may be required by an industry sponsor or the NIH. If not, please click “Not Applicable.”
- Once you’ve completed the registration process, your courses will appear on your home screen. Please start the course by clicking on the title.
Starting a New Course
- The required courses will be listed on your homepage under “Brigham and Women’s Hospital Courses” or “Massachusetts General Hospital Courses.”
- If you previously registered with CITI and no course is listed, please click your institution and click “Add a Course.”
- Select the option “Will you be working with humans (IRB courses)?”
- On the next screen, there are 2 options
- If you have not taken the Basic Course, please choose “Biomedical Research” (1st option) and, if required, please also select “Good Clinical Practice Course.”
- If you have previously completed the Basic Course and need to complete the refresher course, please select that option (3rd option).
- The courses should now appear in your account.
- To start, please click on the course listed.
- Before completing any of the course modules, please complete The Integrity Assurance Statement – click the link listed on the course’s homepage. Once you have read through the statement and agreed to the terms of service, please click “submit.”
- You are now ready to begin the course. Please click on the first module listed on the page and follow the instructions to proceed through the exam. To complete the course, you must complete all the modules and post an average score of at least 80% on all quizzes within the course.
- If you previously registered with CITI and no course is listed, please click your institution and click “Add a Course.”
Completing a Refresher Course/Exam
- If you have completed the Biomedical Research course in years past, you will be asked to complete a “refresher course.”
- If you have taken the BASIC exam, click on the Biomedical Research Refresher 101.
- If you have taken the Refresher 101 exam, please click on the Biomedical Research Refresher 200.
- If you have taken the Refresher 200 exam, please click on the Biomedical Research Refresher 201.
- Before completing any course work, please first complete The Integrity Assurance Statement – click the link listed on the course’s homepage. Once you have read through the statement and agreed to the terms of service, please click “submit.”
- Social and Behavioral Course and Good Clinical Practice Course: If you’ve previously taken the Social and Behavioral course and the Good Clinical Practice Course and need a Refresher, choose Social and Behavioral Refresher and Good Clinical Practice Refresher. Please be sure you’ve already completed the Social and Behavioral and/or Good Clinical Practice BASIC exam. Instructions for these courses are the same as outlined above.
The Notice of Award (NoA) will be sent in full to the Principal Investigator (PI). It is the PI’s responsibility to review the NoA in full. The NoA contains information related to project period dates, total amount of award, information on NIH acknowledgements in publications, press releases etc., award finance information, and terms and conditions. Click the button for a sample Notice of Award.
An advance fund allows spending on an awarded grant while the agreement is being reviewed and executed. If the BWH Department of Surgery (DOS) is a subaward the advance fund allows spending while the Principal Investigators (PIs) and Grants Administrators (GA) are either waiting for the prime institution to send the subaward agreement or while the agreement is being reviewed by MGB Contracting.
Please note that any human subjects (IRB) or animal (IACUC) restrictions apply to advance funds. This means that no human subject or animal related work can be performed, and related expenses cannot be charged to the advance fund until the appropriate approval(s) are in place.
Overview
For PRINCIPAL INVESTIGATORS when BWH DOS is the prime institution:
- Notify your GA that you would like to request an advance fund. Work with them to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Forward a copy of the award notice or email from the sponsor to the GA.
- Approve the request in Insight.
- Forward a copy of the approved IRB or IACUC protocol(s) if appropriate.
For PRINCIPAL INVESTIGATORS when BWH DOS is a subaward institution:
- Notify your GA that you would like to request an advance fund. Work with them to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Forward documentation from the prime institution confirming the award. Documentation may include a copy of the NOA or an email from the PI or GA at the prime institution.
- Approve the request in Insight.
- Forward a copy of the approved IRB or IACUC protocol(s) if appropriate.
For GRANT ADMINISTRATORS when BWH DOS is the prime institution:
- Request a copy of the award notice or email from the sponsor from the PI. You may also receive a copy of the award notice from MGB Research Management (RM).
- Review award notice for any changes in the terms and/or funding amount. Work with the PI and RM to address.
- Work with the PI to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Create the request in Insight, upload any relevant documents, and route for approval.
- Link any IRB or IACUC approvals if appropriate.
For GRANT ADMINISTRATORS when BWH DOS is a subaward institution:
- Ask the BWH PI to forward a copy of any correspondence they have received from the prime institution confirming the award.
- Work with the prime institution to confirm if there any changes to the subaward terms or funding amount. Work with the PI and RM to address.
- Work with the PI to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Create the request in Insight, upload any relevant documents, and route for approval.
- Link any IRB or IACUC approvals if appropriate.
Once RM receives the official award notice or when the subaward agreement is fully executed, the Post-Award Administrator will update the status of the fund to “Active” and the budget details will become visible in Insight.
An advance fund allows spending on an awarded grant while the agreement is being reviewed and executed. If the BWH Department of Surgery (DOS) is a subaward the advance fund allows spending while the Principal Investigators (PIs) and Grants Administrators (GA) are either waiting for the prime institution to send the subaward agreement or while the agreement is being reviewed by MGB Contracting.
Please note that any human subjects (IRB) or animal (IACUC) restrictions apply to advance funds. This means that no human subject or animal related work can be performed, and related expenses cannot be charged to the advance fund until the appropriate approval(s) are in place.
Overview
For PRINCIPAL INVESTIGATORS when BWH DOS is the prime institution:
- Notify your GA that you would like to request an advance fund. Work with them to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Forward a copy of the award notice or email from the sponsor to the GA.
- Approve the request in Insight.
- Forward a copy of the approved IRB or IACUC protocol(s) if appropriate.
For PRINCIPAL INVESTIGATORS when BWH DOS is a subaward institution:
- Notify your GA that you would like to request an advance fund. Work with them to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Forward documentation from the prime institution confirming the award. Documentation may include a copy of the NOA or an email from the PI or GA at the prime institution.
- Approve the request in Insight.
- Forward a copy of the approved IRB or IACUC protocol(s) if appropriate.
For GRANT ADMINISTRATORS when BWH DOS is the prime institution:
- Request a copy of the award notice or email from the sponsor from the PI. You may also receive a copy of the award notice from MGB Research Management (RM).
- Review award notice for any changes in the terms and/or funding amount. Work with the PI and RM to address.
- Work with the PI to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Create the request in Insight, upload any relevant documents, and route for approval.
- Link any IRB or IACUC approvals if appropriate.
For GRANT ADMINISTRATORS when BWH DOS is a subaward institution:
- Ask the BWH PI to forward a copy of any correspondence they have received from the prime institution confirming the award.
- Work with the prime institution to confirm if there any changes to the subaward terms or funding amount. Work with the PI and RM to address.
- Work with the PI to identify a sundry fund that will be charged if the agreement is not fully executed for any reason.
- Create the request in Insight, upload any relevant documents, and route for approval.
- Link any IRB or IACUC approvals if appropriate.
Once RM receives the official award notice or when the subaward agreement is fully executed, the Post-Award Administrator will update the status of the fund to “Active” and the budget details will become visible in Insight.
JUST-IN-TIME
- Update NIH Biosketch and Other Support Page and provide to Grant Administrator (GA).
- Provide current CITI training certificates.
- Provide current IRB and/or IACUC approval(s) if needed.
- Provide any other required documents.
AWARD ACCEPTANCE
- Review award notice for any changes to terms and/or funding levels.
- Work with GA on any changes needed (e.g., adjustments to effort levels or subaward funding levels if funding was reduced).
- Provide final approval so GA can communicate changes to Research Management (RM) and proceed with award acceptance and setup.
AWARD SETUP
Work with GA to identify a sundry fund to be used as back-up for advance fund requests.
- Provide approval for use of the sundry fund.
JUST-IN-TIME
- Assist PI with any updates to their NIH Biosketch and/or Other Support Page.
- Forward all required documents to Research Management (RM). Documents include:
- NIH Biosketch
- Other Support Page
- IRB/IACUC approval
- CITI training certificates
- If DOS is the prime, work with subaward institutions to gather required documents and forward to RM.
- If DOS is a subaward, forward all required documents to the prime institution contact.
AWARD ACCEPTANCE
- Review award notice for any changes to terms and/or funding levels.
- Work with PI on any changes necessary (e.g., adjust effort levels if funding was reduced).
- Communicate any changes to RM so Insight record can be adjusted accordingly.
- If DOS is prime, verify subaward details with RM so agreements can be issued.
AWARD SETUP
- Submit the advance fund request in Insight if necessary.
- Collect and submit/upload updated documents (IRB/IACUC approval, CITI training certificates) if updated versions are needed.
- Submit personnel transactions (EDCs, Earnings Distribution Change; or RPOs, Research Post Only).
JUST-IN-TIME
- Collect all required documents (NIH Biosketches, Other Support Pages, etc.) and review for completeness and accuracy.
- Notify GA and PI of any missing documents or edits that need to be made to submitted documents.
- Conduct final review and submit required documents to the program officer (PO) or prime institution if BWH is the subaward institution.
AWARD ACCEPTANCE
- Review Notice of Award for any changes to terms and conditions and/or funding level.
- Work with GA on reviewing and entering any changes to effort and/or funding levels in Insight.
- Provide official institutional acceptance of the award.
AWARD SETUP
- Review and approve any advance fund requests.
- Enter any budget line changes in Insight.
- Set up deliverables in Insight.
- Establish fund. If there is an advance fund, convert it to the actual fund once the NOGA is received.
DOD OTHER SUPPORT
The Department of Defense (DOD) Other Support document is also known as the “Previous/Current/Pending Support” document and is submitted at the proposal stage.
- For previous awards list all awards with a period of performance ending within the past 5 years.
- Each entry must include:
- Title
- Time commitment
- Supporting agency
- Name and address of the funding agency’s contracting/grants officer
- Performance period
- Level of funding
- Brief description of the project’s goals
- List of specific aims
- Overlap between the proposed project and current and pending research projects. Clearly state if there is no overlap.
- If the investigator has no previous, current or pending support, create a document and enter “None.”
- This document does not have a page limit and is uploaded as “Support_LastName.pdf.”
- Currently, the DOD also allows the use and submission of the National Institutes of Health (NIH) biosketch in place of a DOD formatted biosketch. Always be sure to check your specific grant guidelines and Request for Abstracts (RFA) for exceptions.
NIH OTHER SUPPORT
The information provided below is provided by MGB based on current NIH policy. You can contact your MGB pre-award administrator in addition to your BWH DOS grant administrator if you have any questions about the Other Support document.
Other Support (OS)
-
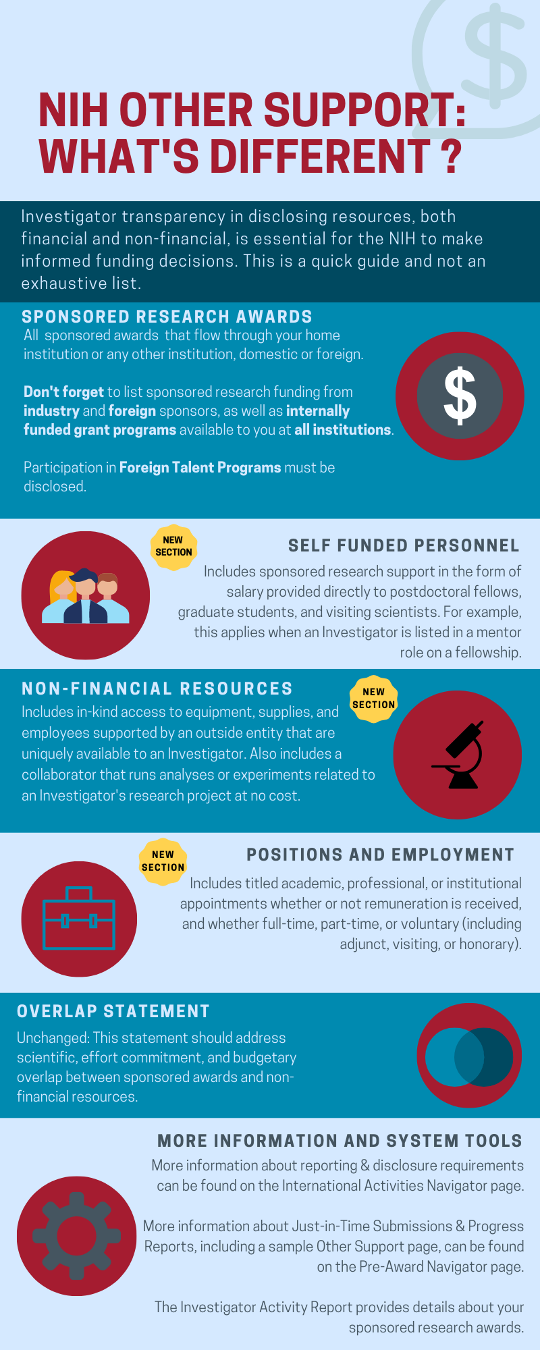
- Although similar to the “Research Support” section of a biosketch, the OS document includes all resources that are available to a researcher to support their research endeavors. This includes non-financial resources (see MGB infographic at the end of this section). The “Research Support” section of the biosketch highlights the investigator’s scientific accomplishments and does not include person months or budget amounts.
- The OS document is submitted during:
- Just-in-Time (JIT) process (Current and Pending Support);
- Progress report (Active Support – do not include Pending Support);
- Proposal stage if you are a mentor or co-mentor on a Mentored Career Development Award.
- Components of the OS Document
- Funding & Awards
- All domestic and foreign funds from local, state, and federal agencies; private associates, non-federal agencies; foundations; industry; other domestic institutions; and funds from foreign governments, institutions, and organizations.
- Research support funding from domestic or foreign entities other than the applicant institution.
- Start-up packages provided by the PI’s home institution are not required to be listed. Start-up packages provided by other institutions are required to be listed.
- Internal grant programs (e.g., Brigham Research Institute funding) should be listed.
- Industry sponsor gifts with a scope of work (SOW).
- Participation in foreign talent programs.
- Award amounts should be listed in total cumulative amounts, including out (projected) budget years.
- Funding & Awards
-
- Self-Funded Personnel
- Self-funded personnel includes research support in the form of salary provided directly to a visiting scientist or postdoctoral fellow who will be devoting effort to your research.
- Non-Financial Resources
- Non-financial resources may be held at your MGB institution or an outside institution.
- Includes access to equipment, supplies, employees or trainees supported in-kind by an outside entity that are uniquely available to the investigator.
- Includes support provided by domestic and foreign entities available in direct support of the investigator’s research efforts. Examples include experiments run by a collaborator at no cost, an instrument loan by a for-profit company to the investigator at no cost, or an additional research lab at another domestic or foreign institution held by an investigator with multiple positions.
- Position and Appointments
- All domestic and foreign positions and scientific appointments. All academic, professional, or institutional appointments should be listed, including whether remuneration is received or not, and whether the appointment is full-time, part-time, or voluntary.
- Overlap Statement
- The overlap statement should address the actual or perceived overlap between sponsored awards and/or non-financial resources, including scientific, effort, and budgetary.
- Self-Funded Personnel
Post Award
Prior to the start of any sponsored research project (federal or non-federal), the Principal Investigator (PI) and Department Grants Administrator (GA) should review the sponsor guidelines, the Office of Management and Budget’s (OMB) Uniform Guidance cost principles, Mass General Brigham (MGB) policies, and the Notice of Award (NOA) for any expenditure stipulations.
MGB cost charging information can be found on the Research Navigator site (links below):
For NIH-sponsored research awards, refer to section 7.9.
Allowability of Costs/Activities
Remember that the budget is the financial expression of the research project!
All costs charged to a sponsored research agreement must be:
- Allowable
- Allocable
- Reasonable
- Consistently applied
Note: Unallowable costs “whether originally budgeted or erroneously charged” must be transferred to the appropriate fund. Refer to the MGB Cost Transfer Policy for more details.
MGB Research Management (RM) has outlined the following scenarios to AVOID:
- Assigning costs to awards based on the remaining balance.
- Assigning costs to an award before the cost is incurred.
- Assigning costs to an award that receives no benefit from the activity.
- Assigning costs to an award that are part of regular administrative support for hospital activities.
- Charging an expense exclusively to one award when the expense supported other or multiple awards.
- Charging the budgeted amount rather than the actual cost.
- Rotating charges without a rotation schedule that reflects the benefit to each project.
The PI is responsible for ensuring that research staff are also trained and informed on cost charging principles. The following sample activities require an understanding of allowable/unallowable expenses on sponsored research awards:
- Ordering lab or office supplies, equipment, computers, telecommunication, etc.
- Approving requisition purchase orders
- Submitting and approving an expense (reimbursement) report
- Booking travel, business meal, event, etc.
- Registering for conferences, membership, etc.
- Approving invoices for payment (subcontract, consultant, publication, vendor, tuition or stipend, etc.)
- Transferring costs and open purchase orders
The PI should contact the GA if there are any questions or if further clarification is needed.
Progress reports are typically submitted on an annual basis and include updates on the scientific/technical progress of the project, as well as information on spending and carry-forward requests. Progress reports may be submitted electronically (e.g., via eRA Commons for National Institutes of Health [NIH] grants) or via email to the agency contact.
Below are the typical responsibilities for the Principal Investigator (PI), Grants Administrator (GA), and Mass General Brigham (MGB) Research Management for progress reports:
PRINCIPAL INVESTIGATOR
- Forward any notifications/reminders that a progress report is due to your GA.
- Provide required scientific/technical information to the project manager and GA.
- For NIH grants, assist GA with language for justification if carry-forward balance exceeds 25%.
- Review information entered for completeness and accuracy. Provide final approval of content.
- Route NIH progress reports in eRA Commons to the MGB pre-award administrator (authorized institutional official). NOTE: This step can only be done under your login even if you have delegated authority to the project manager or GA.
GRANTS ADMINISTRATOR
- If an NIH Progress Report, initiate the progress report in eRA Commons or appropriate agency mechanism specific to the Sponsor.
- Work with the PI and/or project manager to gather the required scientific and project information.
- Work with the MGB Post-Award Administrator to determine carry-forward balance and work with PI to prepare justification if it exceeds the agency threshold (e.g., >25% for NIH grants).
- Review that all information entered is accurate and complete. (NOTE: The PI is still responsible for providing final approval and sign-off.)
- Complete the progress report in eRA Commons and notify PI once it is ready for approval and routing.
- Follow up with MGB Pre-Award Administrator to ensure that the progress report is submitted in a timely fashion.
RESEARCH MANAGEMENT
- Confirm carry-forward balance (Post-Award).
- Review information entered and any attachments provided for completeness and accuracy.
- Provide institutional sign-off.
- Submit to the agency when required/appropriate (Pre-Award).
The Subrecipient Monitoring requirement applies to federal and non-federal projects and continues throughout the lifetime of the award. Responsibility for financial and administrative monitoring of collaborators is shared among the Principal Investigator (PI), Department/Grants Administrator, and Mass General Brigham (MGB) Research Management staff. Responsibility for technical monitoring resides solely with the PI.
All MGB subrecipient monitoring information can be found on the Research Navigator site, links below.
Additional BWH Department of Surgery (DOS) Guidance
Subrecipient Invoice Approvals
The DOS Grants Administrator will provide cursory review of subcontract invoice and will notify the PI to complete final review. Cursory review includes (1) verifying expenses are allowable, allocable, and reasonable, and incurred within sub-agreement budget period per the sub-agreement and the prime award; (2) ensuring direct costs, indirect costs, and cumulative expenses are calculated and totaled correctly; and (3) ensuring the invoice dates are within the sub-agreement period.
The PI should correspond with the Grants Administrator prior to approving the invoice. The PI has 20 days (from the initial Insight email notification) to approve in Insight or alert the Grants Administrator to approve on PI’s behalf. Any objection must be communicated within this timeframe to the Grants Administrator and MGB Research Finance. The Grants Administrator or PI will reach out to the administrative contact at the subrecipient to resolve any discrepancies on the invoice.
Compliance
It is the PI’s responsibility to ensure that the subcontracts, including any third parties, have the appropriate IRB and IACUC approvals from their institutions (if ceded review), BWH, MGB, and/or the funding agency according to terms and conditions of the award. The PI should work with the Grants Administrator and MGB Research Management Post-Award to ensure approvals are received prior to performing work. Non-compliance may result in withholding of funds and/or termination of the award.
The Principal Investigator (PI), study staff, DOS Research Administration team, and MGB Research Management all have different sponsor billing responsibilities.
It is the policy and responsibility of MGB Research Management to invoice on behalf of the PI and research team on federally-funded and non-profit sponsored research projects monthly. For the majority of industry-funded clinical trials, the responsibility of invoicing falls to the division/department, specifically the DOS Research Administration team/grants manager.
Regardless of the project origin or Sponsor type, PIs have the primary responsibility for reviewing and confirming all obligations and expenses posted to the fund before generating an invoice.
PI responsibilities include:
- Reviewing fund balances of the project to validate the allowability, allocability, and reasonableness of all costs incurred during the project’s life. (See MGB Research Management’s Sponsored Project Cost Charging Policy)
- Identifying expenses posted to the fund in error.
- Working with the Grant Administrator (GA) on transferring expenses to/from the fund per the MGB Research Management’s Cost Transfer Policy.
- Assisting with the reconciliation of projects with multiple fund numbers to balance appropriate expenses with funding for each fund number.
- Providing additional information necessary to meet the financial reporting requirements of the award.
- Reviewing and approving the final financial report created by Research Finance.
- Ensuring that all technical deliverables are met in keeping with payment milestones and other requirements.
Department Administration responsibilities include:
- Providing regular financial reports for PI to review in preparation for billing/invoicing.
- Assisting the PI with the review of all costs incurred to confirm their allowability, allocability, and reasonableness.
- Assisting the PI with any cost transfer requests.
- Providing MGB Research Finance with additional documentation necessary to complete and submit the invoice when required.
- Assisting the PI with the review of the final invoice created by MGB Research Management.
- Assisting MGB Research Finance in facilitating payment from sponsors for outstanding receivables, when required.
- Maintaining detailed back-up of the project’s activities supporting the costs reflected on the invoice, when required.
MGB Research Finance responsibilities include:
- Preparing, reviewing, and submitting the invoice.
- Obtaining all payments from sponsors for outstanding receivables.
- Providing final confirmation that project expenses are allowable, allocable, and reasonable.
- Providing the PI/ department with a copy of the final invoice.
- Maintaining the official institutional record of invoices generated by MGB Research Management.
Additional BWH Department of Surgery (DOS) Guidance
Effort is the amount of time devoted to a project. It is an area of scrutiny by sponsors because personnel expenses average 60% of research budgets. In the Post-Award stage, the PI is responsible for meeting his/her own “committed” effort on the grant proposal, and for ensuring that the research personnel also meet their committed effort on sponsored grants. This applies to any research staff outside of BWH DOS; the PI and Grants Administrator (GA) should collaborate with the other department’s GA to review/confirm actual effort on a regular basis. It is crucial that the PI direct the GA on any effort changes for the PI and for research staff during monthly review of effort and allocations on all funds.
During progress report submissions, the PI and GA will also report personnel changes in effort of senior/key personnel designated in the Notice of Award and verify that effort matches the current effort commitments.
Effort Changes
Once it has been decided that allocation needs to be adjusted to reflect actual effort (either retroactively or future), the GA will send an email request to the appropriate PeopleSoft tree manager of the PI or staff member to submit an EDC (Earnings Distribution Change) or RPO (Research Post Only) for retroactive changes. The following should be provided to the EDC/RPO submitter:
- Change type: EDC or RPO
- Personnel name
- Effective date
- End date if applicable (note: cannot enter a future end date)
- Percentage (%) changes and business unit/fund numbers (i.e. 2200/123456)
- Brief comment about the proposed changes
- This comment must include language confirming salaries on sponsored research funds (or the salary allocation) reflect actual or anticipated (for future dated EDCs) effort. If there is a discrepancy between salary and effort, the comment must clarify what the actual effort is in relation to the salary charged.
MGB discourages over-90-days changes in effort allocations on sponsored research funds; it may, however, be necessary in some cases. All retroactive data changes greater than 90 days should include the phrase, “The data change is greater than 90 days because…” followed by justification that answers the five mandatory compliance questions. The following types of EDC/RPOs do not require the > 90-day justification, however, comments explaining the transaction are always required.
- A personnel transaction transferring salaries to a discretionary fund.
- A salary allocation placing salary on a newly established fund retroactive greater than 90 days, but submitted within 90 days of fund setup. Department should state the activation date in the comments field.
In many cases, any significant effort changes (i.e. greater than 25% from original committed effort) will require prior approval from the sponsor. The PI and GA should work with MGB Research Management Post-Award to submit a formal request to the sponsor. Once approved by the sponsor, the budget forms, Other Support, and personnel reports should be updated to reflect the change; approval documentation should be saved in DropBox and Insight.
Salary Cost Sharing
In general, during the Pre-Award budget stage, a PI should request equivalent salary dollars for proposed effort on sponsored projects. In some cases, a PI may elect to cost-share the proposed effort. Cost sharing is defined as the portion of a project or program cost not reimbursed by the sponsor. Cost sharing may also be referred to as in-kind contributions or matching funds. MGB discourages cost sharing on sponsored projects unless it is specifically mandated by the sponsor as a condition of the award. Any salary cost sharing will need to be monitored and tracked as part of effort management.
Additional DOS Guidance
As of FY21, the DOS will cover the cost sharing variance for any PI whose Institutional Base Salary (IBS) exceeds the NIH salary cap on NIH grant awards only. During Pre-Award and Peri-Award stages, the GA should contact Jamie Fu and/or Matt Sandler from BWPO Finance to confirm fund number. During Post-Award stage, the GA should work with BWPO Finance to track cost sharing effort, and review with the PI on a monthly basis. For cost sharing on non-NIH grants (either due to grant limitation or voluntary), the PI is responsible for identifying a non-sponsored fund to cover the variance if the grant is awarded.
Effort Reporting and Certification
PIs are responsible for verifying the appropriateness of all charges to sponsored projects, particularly those for compensation of personnel services since they generally represent significant expenditures. Semi-annual effort certification is required in October and April. PIs are encouraged to take the Electronic Effort Reporting for Investigators on HealthStream.
Closeout refers to the process that takes place when a grant or contract is ending. The closeout process involves submitting final progress/technical reports, the Final Financial Report (FFR), and, in some cases, invention reports.
Below are the typical responsibilities for the Principal Investigator (PI), Grants Administrator (GA), and MGB Research Management (RM) for closeouts:
PRINCIPAL INVESTIGATOR:
- Submit all required scientific/technical information for the final progress report.
- Review all expenses for allowability, allocability, reasonableness, and consistency.
- Review personnel effort levels for the FFR and/or final invoice.
- Complete the Invention Report and send to GA.
- Review all reports for complete accuracy. Provide final approval of all reports via email or Insight.
- Work with GA to re-allocate all personnel funded on the project closing to another funding source.
- Approve final invoices to the sponsor or prime institution.
- Inform all research staff to stop spending on the fund.
GRANTS ADMINISTRATOR:
- Review fund to ensure that all salaries and expenses have posted to the fund before the FFR and/or final invoice is generated and sent to the sponsor.
- Review fund with the PI.
- Work with the PI and/or research staff to ensure all encumbrances (e.g., open purchase orders) are closed out or transferred to another fund.
- If BWH is the prime institution, ensure that all subaward institutions have submitted final invoices by the deliverable date specified in the subaward agreement.
- If BWH is the subaward institution, ensure that all salaries and expenses have posted before the final invoice is sent to the prime institution.
- Submit all necessary cost transfers or journal entries.
- Ensure that all salaries are moved off the fund via EDC (Earnings Distribution Change)/RPO (Research Post Only) requests before the project end date/salary controls take effect.
MGB RESEARCH MANAGEMENT:
- Process all EDCs/RPOs, cost transfers, and journal entries.
- Review and submit all final reports (technical, financial, invention).
- Generate FFR and final invoices and send to PI/GA for approval.
- Submit approved invoices to the sponsor or prime institution.
- Update Insight fund status to “Inactive.”
Although MGB Research Finance Accounts Receivables is responsible for collecting payment of invoices and drawdown of funds, the GA should also monitor the revenue amounts in Insight Financial Reports.
FINANCIAL REPORTING
- Generate monthly or quarterly financial reports for all funds, including non-research and research sundry funds.
- Review reports with Principal Investigator (PI) and research staff.
- Complete and submit any salary changes in a timely manner. For MDs, email any salary changes to David Breen in BWPO for submission before the monthly deadline.
- Submit cost transfers and journal entries in a timely manner.
- Alert PI if current spending trend will result in a >25% carryforward for NIH grants or for the applicable agency threshold. Assist with preparing justification for the carryforward request.
- Complete and submit requests for new research and non-research sundry funds.
EFFORT REPORTING
- Complete quarterly internal effort report and review with PI/research staff.
- Make any necessary effort adjustments for all research personnel.
- Complete biannual MGB effort reporting and route for certification.
- Track salary cost-sharing for PIs if applicable.
PROGRESS REPORTS
- Initiate progress report in eRA Commons for NIH grants or the appropriate agency mechanism for non-NIH grants.
- Work with PI and/or Project Manager to gather required scientific and project information.
- Collect information from subcontracts and collaborators.
- Work with the MGB Post-Award Administrator to determine carryforward balance, and work with PI to prepare justification if it exceeds the agency threshold (e.g., >25% for NIH grants).
- Review that all information entered is accurate and complete. NOTE: The PI is still responsible for providing final approval and signoff.
- Complete progress report in eRA Commons and notify PI once it is ready for approval and routing.
- Follow up with MGB Pre-Award Administrator to ensure that the progress report is submitted in a timely fashion.
CLOSEOUT
- Ensure that all salaries charged to a fund are moved off before the project end date/salary controls take effect via EDC (Earnings Distribution Change)/RPO (Research Post Only) requests.
- Ensure that all expenses are allowable, allocable, and reasonable, and have posted to the fund before a final invoice is generated and sent to the sponsor.
- Work with PI and/or research staff to ensure all encumbrances (e.g. open purchase orders) are closed out or transferred to another fund.
- In instances where BWH is the prime institution, ensure that all subawards have submitted final invoices and other required reports before the sponsor deadline.
- In instances where BWH is the subaward institution, ensure that the final invoice is sent to the prime institution by the deadline.
FINANCIAL REPORTING
- Generate financial reports and send them to PI/GA for review and approval.
- Make any changes requested by the GA.
- Provide final approval and sign-off.
- Submit final and signed financial report to sponsor.
EFFORT REPORTING
- Make any changes to effort requested by GA.
- Make historical edits to effort reports (e.g., the fund is established after the reporting window ends so effort was not entered at that time). NOTE: This may not be Research Management, but it is done at the MGB-level.
PROGRESS REPORTS
- Work with GA to ensure that carry forward balance information is accurate and assist with language if carry forward amount is >25% for NIH grants (Post-Award).
- Review information entered by GA and/or PI for completeness and accuracy.
- Conduct final review and provide institutional sign-off.
- Submit progress report to sponsor.
CLOSEOUT
- Approve all requests to remove salaries from funds that are ending.
- Ensure that all pending invoices, including from subaward institutions if BWH is the prime, have been paid before the fund is closed and the final financial report is sent to the sponsor.
- Work with GA on closing out open POs and other encumbrances.
- Review, approve, and submit any final progress and/or technical reports depending on sponsor requirements.
The following requests may require prior approval from the sponsor or pass-through entity (PTE). The Principal Investigator (PI) should notify the Grants Administrator (GA) as soon as possible. The GA will work with Mass General Brigham Research Management (MGB RM) Post-Award to review the award terms and conditions, inquire with the sponsor, and determine if a formal request is required.
- Carry-forward unobligated balance
- Re-budgeting
- Changes in the Scope of Work (SOW)
- No-Cost Extension (NCE)
- Changes in effort greater than 25%
- Changes in PI or Key Personnel
- Addition or removal of a subcontract
If a formal prior approval request is required:
- The PI and GA will draft a formal letter/e-mail, and send it to MGB RM Post-Award.
- Include any back-up documentation (i.e. budget, justification, etc.)
- MGB Post-Award will review, co-sign, and submit to sponsor.
If a formal request or approval is not required:
- The GA will work with MGB RM Post-Award to ensure that the request is documented and updated in Insight.
The National Institutes of Health (NIH) No-Cost Extension (NCE) is available to Principle Investigators (PIs) who were awarded a grant under the NIH’s Standard Terms of Award. Per the NIH, the “NIH Standard Terms of Award include the provision for grantees to extend the final budget period of a previously approved project period one time for a period of up to 12 months, without additional NIH funds, and without prior approval.” This action is known as an NCE. The request for an NCE must be submitted before the project period ends.
Process and Responsibilities
Principle Investigator (PI)
The PI is responsible for determining the need and appropriateness of an NCE. An NCE should only be used under at least one of the following NIH defined criteria:
- Additional time beyond the established expiration date is required to ensure adequate completion of the originally approved project.
- Continuity of NIH grant support is required while a competing continuation application is under review.
- The extension is necessary to permit an orderly phase-out of a project that will not receive continued support.
It should be noted that having unspent funds at the end of a project does not, in and of itself, constitute a valid justification for an NCE.
The NIH goes on the define additional parameters which must be met for an NCE to be deemed appropriate:
- No additional funds are required from the NIH awarding office.
- There will be no change in the project’s originally approved scope.
- There is nothing in the award notice/terms of award that specifically precludes an extension.
Grant Administrator (GA)
It is the responsibility of the GA to alert the PI well in advance of the grant’s original project period end date to allow the PI enough time to determine if an NCE is appropriate. The NCE should be submitted within 90 days of the project period’s original end date.
The GA also acts a liaison between the PI and Mass General Brigham (MGB) Research Management (RM). Once the PI has determined to move forward with the NCE the GA should contact their MGB RM Post-Award Administrator. Only the Signing Official (SO) can request an NCE under the eRA Commons Prior Approval function.
MGB Research Management (RM)
The Post-Award GA is often a designated NIH SO and is responsible for submitting an NCE request. When requesting an NCE, please provide the Post-Award GA with the proposed length of the extension (1-12 months).